Online-Seminar: In-vitro performance testing for topically applied formulations
with a focus on the adopted „Guideline on quality and equivalence of locally applied, locally acting cutaneous products“ becoming effective April 2025
Kursnummer: 7045
- Liquid Dosage Forms
- Research and Development
- Semi-solid Dosage Forms
Kursprogramm / Flyer
- 7045_IVPT.pdf (906 KB)
Beginn:
04.02.2025, 13:00 Uhr
Ende:
04.02.2025, 17:00 Uhr
Termin zum Kalender hinzufügen
Zusammenfassung
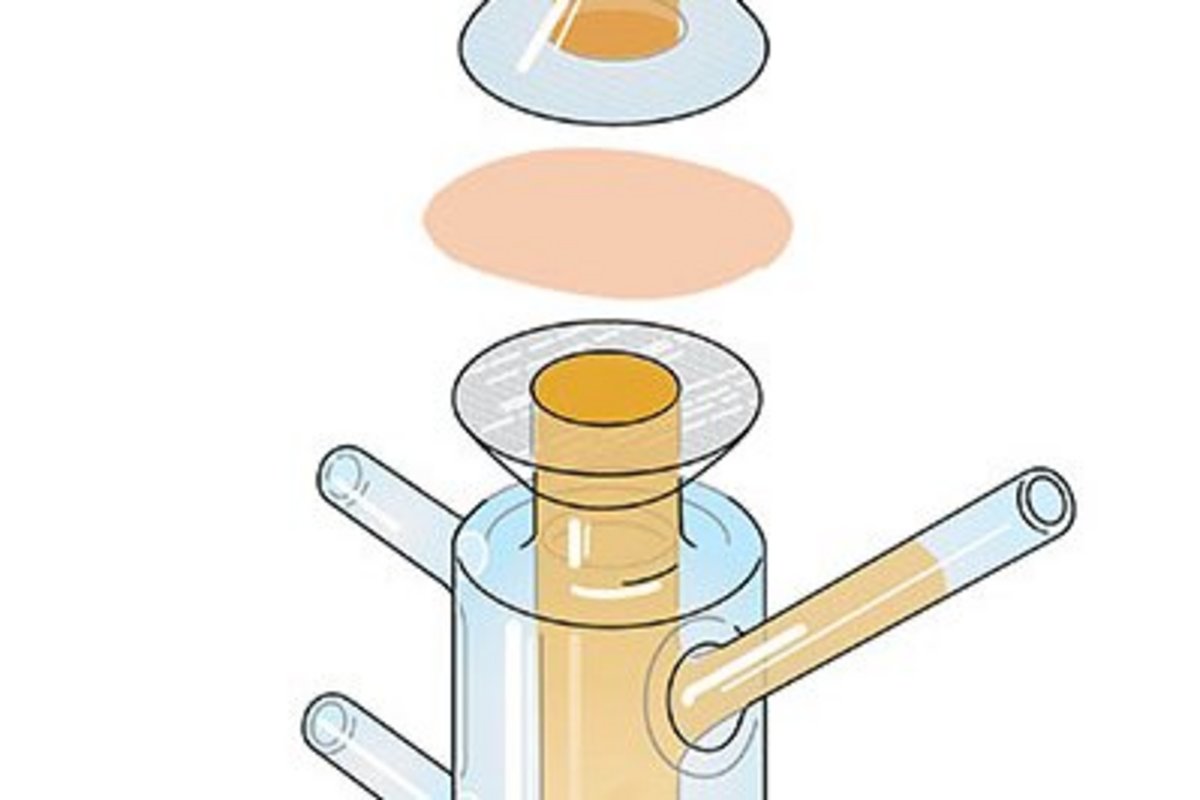
In order to be effective, topically applied drugs need to overcome at least the outermost layer of the skin. The skin is a complex and efficient barrier with various factors that can influence penetration. Paradigms of dermal bioavailability are different and at least partially less straightforward than in peroral drug delivery. And, there is not the single in vitro model that fits all purposes. Nevertheless, in vitro performance testing of topicals can be performed in a systematic manner. It is an important aspect of early projects for compound and formulation selection and overall program de-risking, and can support toxicological characterization. Most importantly, it is used to establish bioequivalence in generic development programs and post-approval changes. For this purpose, the respective regulatory guidelines must be taken into account.
Zielgruppe
The course is designed for all scientists involved in In-Vitro Permeation testing and In-Vitro Release Testing of topically applied formulations.
Zielsetzung
This online course provides a comprehensive overview of in vitro performance testing by academic and industry experts. It begins with an introduction to skin anatomy, principles of dermal pharmacokinetics, and a general overview of release and penetration models. In vitro release testing (IVRT), an important model for demonstrating quality and equivalence of semi-solids, is introduced, as is in vitro permeation testing (IVPT), which is used for equivalence testing as well as exploratory development and toxicology. Finally, some important information on differences between draft guideline and final guideline will be discussed.
Veranstalter
Kurfürstenstraße 59
55118 Mainz
Deutschland
Dr. Martin Bornhöft
Tel.: +49 6131 9769-0
E-Mail: info@apv-mainz.de
https://www.apv-mainz.de
Preise
Alle Preise mehrwertsteuerfrei gemäß § 4,22 UStG